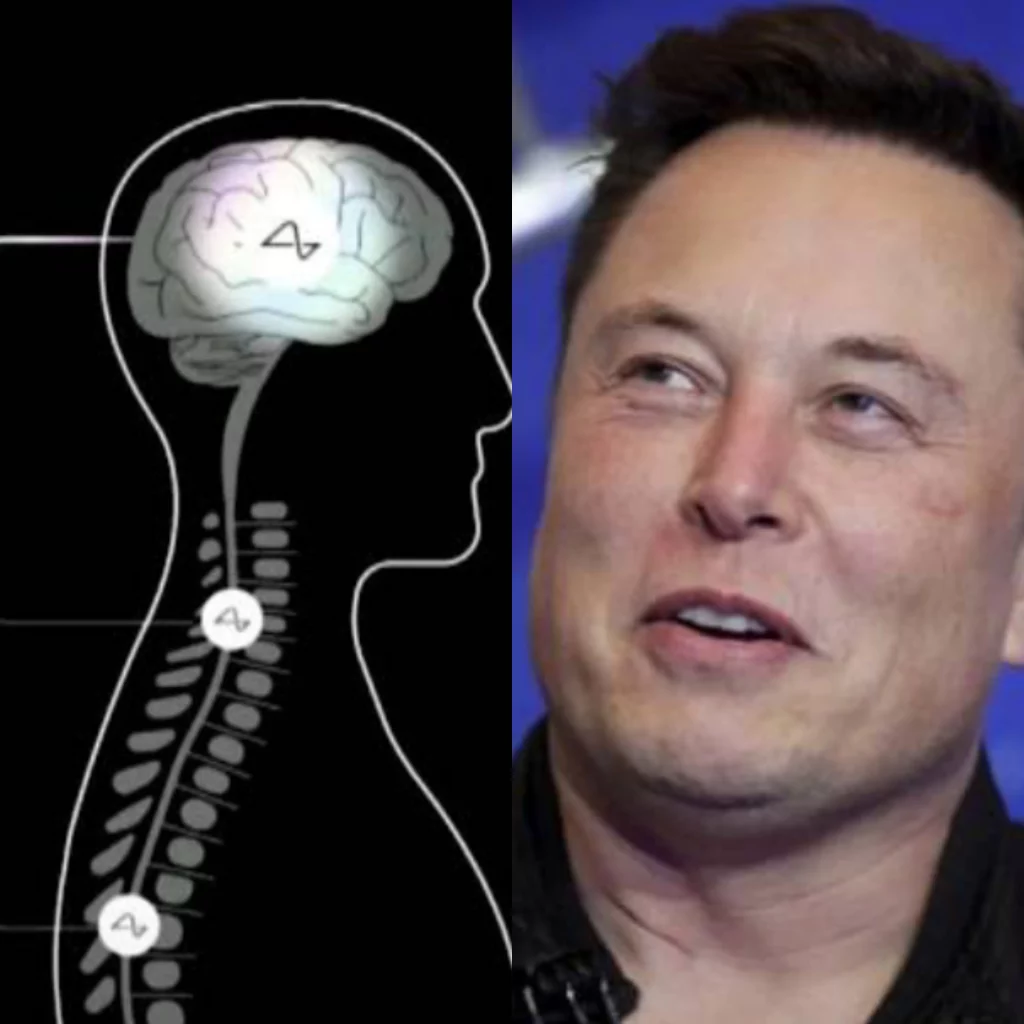
US Rejected Elon Musk’s Bid to Test Brain Chips on Humans that Could Reportedly Cure Blindness and Paralysis
The US government reportedly rejected plans by Elon Musk’s company Neuralink to test Brain chips on humans that could ‘help make the blind to see’ citing some serious safety issues.
Man Dies after Contracting Brain-eating Amoeba from Rinsing Sinuses with Tap Water
Musk had announced that his BCI company, Neuralink would start human trials by November this year.
He shared a video demonstrating the BCI or brain-computer interface that Neuralink was working on. In the video a nine-year-old monkey called Pager with a Neuralink chip inserted in his brain was taught to play Pong with a joystick.
After learning the patterns, the joystick was disconnected and the monkey’s brainwaves were connected to the game and he was able to play it.
The video generated a lot of media coverage with tech pundits claiming that it would revolutionise medicine, and change how humans interacted with computers forever.
According to a new Reuters report, Musk’s Neuralink implants won’t be as widely available and widely adopted as Musk would have wanted it to be as US regulators rejected Musk’s bid to test brain chips on humans, citing safety risks.
The report added that despite being rejected by US health agencies, Elon Musk has forecasted that Neuralink will soon begin human testing of a revolutionary brain implant to cure chronic diseases like paralysis and blindness at least on four separate occasions.
Neuralink’s rejection by the US Food and Drug Administration (FDA) was not previously known to the media.
According to Reuters Musk and Neuralink must resolve dozens of issues before they can begin human testing, a key milestone on the road to final product clearance.
The FDA’s main safety concerns included the device’s lithium battery, the possibility of the implant’s microscopic cables migrating to other regions of the brain, and whether and how the device could be removed without harming brain tissue. Musk was denied as recently as November 2022.
During the hour-long demonstration in November, Musk stated that the company had filed “most of our papers” to the FDA, without naming any formal application. Right after the presentation Neuralink executives falsely claimed that the FDA asked them a few questions on safety, in what they said was an ongoing and open discussion.
Man Dies after Contracting Brain-eating Amoeba from Rinsing Sinuses with Tap Water
Musk had announced that by November 30 this year, Neuralink would get FDA approval and clearance on human trials, if the FDA grants them the required permissions to do so.
Neuralink is still working through the FDA’s concerns almost a year after they were denied human trials the report added. Three employees expressed skepticism that the business could swiftly resolve the problems, especially given the deadline.